What is a bond between a positive and a negative ion called.
If you’re looking for what is a bond between a positive and a negative ion called pictures information linked to the what is a bond between a positive and a negative ion called keyword, you have come to the right site. Our site frequently provides you with hints for seeking the highest quality video and picture content, please kindly hunt and locate more informative video content and graphics that fit your interests.
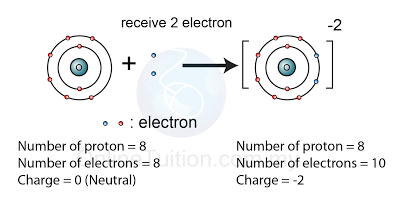 What Is The Difference Between Atoms And Ions And Covalent Compounds And Ionic Compounds Socratic From socratic.org
What Is The Difference Between Atoms And Ions And Covalent Compounds And Ionic Compounds Socratic From socratic.org
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge share CB. A number of ions grouped together in a regular repeating pattern is called a _____ _____ Crystal Lattice. What is the difference between ionic and covalent bond. Ionic bonds are formed because of transfer of electrons from metal to non-metal.
It has a positive charge because it is.
An ionic bond forms between a ____ ion with a positive charge and a ____ ion with a negative charge share CB. Ionic bond is also called as electrovalent bond. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an Ionic Bond. Ion any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations.
 Source: pinterest.com
Source: pinterest.com
This bond is formed between two atoms whose electronegativity difference is large and they can attain octets by the complete transfer. Ions form when atoms lose or gain electrons to obtain a full outer shell. The electrostatic force of attraction between positive ion and negative ion that holds them together is called ionic bond. Is sodium a negative or positive ion. In a covalent bond the atoms bond by sharing electrons.
Metal atoms lose electrons to form positively.
An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom. In a covalent bond the atoms bond by sharing electrons. The key difference between positive and negative ion is that the positive ion carries a positive electrical charge whereas the negative ion carries a negative electrical charge. A bond between two positive ions.
 Source: physics-and-radio-electronics.com
Source: physics-and-radio-electronics.com
When the atom loses one or more electrons it will become a positive ion. The atom that gains one or more electrons or loses one or more electrons is called an ion. By combination of ions with other particles. What type of bond is a positive and negative attraction.
 Source: pinterest.com
Source: pinterest.com
A chemical bond resulting from the electrostatic attractionbetween positive and negative ions is called an Ionic Bond. Ions are chemical species that derive from either the electron loss or gain. A chemical bond resulting from the electrostatic attractionbetween positive and negative ions is called an Ionic Bond. These oppositely charged ions are usually produced when a metal transfers its electron to a nonmetal.
 Source: nl.pinterest.com
Source: nl.pinterest.com
The atom that gains one or more electrons or loses one or more electrons is called an ion. Ion any atom or group of atoms that bears one or more positive or negative electrical charges. An ion is a charged particle such as Na the sodium ion. The electrostatic force of attraction between the oppositely charged ions is called the ionic bond or electrovalent bond.
The unequal sharing of the bonding electrons produces negative and positive ends or poles where electron density is lower the positive pole and higher the negative pole. An ion is a charged particle such as Na the sodium ion. For example when an electron is removed from a neutral sodium atom the sodium atom becomes a positively charged sodium ion Na and when this electron is transferred to a neutral chlorine atom. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds.
A bond between two atoms Sign In.
By combination of ions with other particles. A bond between two atoms Sign In. What is the definition of a covelant bond. Ionic bonds result from transfer of electrons whereas covalent bonds are formed by sharing. Negatively charged ions are called anions.
 Source: pinterest.com
Source: pinterest.com
An ionic bond is a force of attraction between a positive and a negative charged ion. Atoms that are high on the electronegativity scale such as fluorine and oxygen become anions by gaining electrons. Ions form when atoms lose or gain electrons to obtain a full outer shell. In this course metals get positive charge because of transfer of electrons and non-metal gets negative charge because of acceptance of electrons. In other words bond formed between positive and negative ion is called ionic bond.
A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an covalent bond. A bond between two atoms Sign In. Ions form when atoms lose or gain electrons to obtain a full outer shell. In other words bond formed between positive and negative ion is called ionic bond.
Negatively charged ions are called anions.
Covalent bonds usually occur between nonmetals. For example sodium Na a metal and chloride Cl a nonmetal form an ionic bond to make NaCl. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. Ionic bonds result from transfer of electrons whereas covalent bonds are formed by sharing.
 Source: pinterest.com
Source: pinterest.com
Each atom contributes an equal number of electrons towards the bond formation. The unequal sharing of the bonding electrons produces negative and positive ends or poles where electron density is lower the positive pole and higher the negative pole. Ion any atom or group of atoms that bears one or more positive or negative electrical charges. One example of an ionic bond is the formation of sodium fluoride NaF from a sodium atom and a fluorine atom.
 Source: nl.pinterest.com
Source: nl.pinterest.com
An ion is a charged particle such as Na the sodium ion. Ions are chemical species that derive from either the electron loss or gain. When the atom loses one or more electrons it will become a positive ion. Ionic bonds result from transfer of electrons whereas covalent bonds are formed by sharing.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
The unequal sharing of the bonding electrons produces negative and positive ends or poles where electron density is lower the positive pole and higher the negative pole. The unequal sharing of the bonding electrons produces negative and positive ends or poles where electron density is lower the positive pole and higher the negative pole. When the atom loses one or more electrons it will become a positive ion. What is the difference between ionic and covalent bond.
This charge can be either positive or negative.
Ions form when atoms lose or gain electrons to obtain a full outer shell. By combination of ions with other particles. A bond between a positive and negative ion. Another atom typically a non-metal is able to acquire the electrons to become a negative ion or anion. Ionic bonds are formed because of transfer of electrons from metal to non-metal.
 Source: pinterest.com
Source: pinterest.com
This bond is formed between two atoms whose electronegativity difference is large and they can attain octets by the complete transfer. It has a positive charge because it is. The bond is formed when an atom typically a metal loses an electron or electrons and becomes a positive ion or cation. What is the definition of a covelant bond. Ionic bonds result from transfer of electrons whereas covalent bonds are formed by sharing.
Highly electronegative atoms strongly attract electrons thus attaining eight electrons.
Or group of atoms with a positive or negative charge. The atom that gains one or more electrons or loses one or more electrons is called an ion. The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. A positive ion is called _____ Cation.
 Source: pinterest.com
Source: pinterest.com
When the atom loses one or more electrons it will become a positive ion. True or False. Is sodium a negative or positive ion. What is the difference between ionic and covalent bond.
 Source: in.pinterest.com
Source: in.pinterest.com
When the atom loses one or more electrons it will become a positive ion. In this course metals get positive charge because of transfer of electrons and non-metal gets negative charge because of acceptance of electrons. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an Ionic Bond. What is the difference between ionic and covalent bond.
 Source: pinterest.com
Source: pinterest.com
Ionic bonds result from transfer of electrons whereas covalent bonds are formed by sharing. A number of ions grouped together in a regular repeating pattern is called a _____ _____ Crystal Lattice. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an Ionic Bond. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom.
The covalent bond is a bond formed when two atoms share one or more electron pairs.
Negatively charged ions are called anions. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an covalent bond. What is the definition of a covelant bond. The electrostatic force of attraction between the oppositely charged ions is called the ionic bond or electrovalent bond. The resulting charges are called partial positive and partial negative Partial is used because these charges are not as great as the numeric charges we assign to elctrons -1 or protons 1.
 Source: pinterest.com
Source: pinterest.com
In other words bond formed between positive and negative ion is called ionic bond. What is the difference between ionic and covalent bond. For example sodium Na a metal and chloride Cl a nonmetal form an ionic bond to make NaCl. The covalent bond is a bond formed when two atoms share one or more electron pairs. Or group of atoms with a positive or negative charge.
Like next-door neighbors whose kids hang out first at one home and then at the other the atoms do not lose or gain electrons permanently.
Atoms that are high on the electronegativity scale such as fluorine and oxygen become anions by gaining electrons. This charge can be either positive or negative. True or False. Ions are chemical species that derive from either the electron loss or gain.
 Source: in.pinterest.com
Source: in.pinterest.com
The definition of ionic bond is when a positively charged ion forms a bond with a negatively charged ions and one atom transfers electrons to another. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an covalent bond. This bond is formed between two atoms whose electronegativity difference is large and they can attain octets by the complete transfer. For example when an electron is removed from a neutral sodium atom the sodium atom becomes a positively charged sodium ion Na and when this electron is transferred to a neutral chlorine atom. It has a positive charge because it is.
 Source: pinterest.com
Source: pinterest.com
Unlike ionic bonds formed by the attraction between a cations positive charge and an anions negative charge molecules formed by a covalent bond share electrons in a mutually stabilizing relationship. The bond is formed when an atom typically a metal loses an electron or electrons and becomes a positive ion or cation. Ionic bonds are formed because of transfer of electrons from metal to non-metal. The electrostatic force of attraction between positive ion and negative ion that holds them together is called ionic bond. A negative ion is called _____ Anion.
 Source: nl.pinterest.com
Source: nl.pinterest.com
The electrostatic force of attraction between positive ion and negative ion that holds them together is called ionic bond. Each atom contributes an equal number of electrons towards the bond formation. Like next-door neighbors whose kids hang out first at one home and then at the other the atoms do not lose or gain electrons permanently. A chemical bond resulting from the electrostatic attraction between positive and negative ions is called an Ionic Bond. Highly electronegative atoms strongly attract electrons thus attaining eight electrons.
This site is an open community for users to do sharing their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site convienient, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is a bond between a positive and a negative ion called by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





