What is a bond called that shares electrons between two neutral atoms.
If you’re looking for what is a bond called that shares electrons between two neutral atoms pictures information related to the what is a bond called that shares electrons between two neutral atoms keyword, you have visit the ideal site. Our site frequently gives you suggestions for refferencing the highest quality video and image content, please kindly surf and locate more informative video articles and graphics that match your interests.
 How To Calculate Oxidation Number Practice Problems Oxidation State Writing Instruction Organic Chemistry Study From pinterest.com
How To Calculate Oxidation Number Practice Problems Oxidation State Writing Instruction Organic Chemistry Study From pinterest.com
The type of the chemical bond that forms when electrons are shared between atoms that are neutral have no electrical charge is A. These electrons are simultaneously attracted by the two atomic nuclei. Water is an example of a compound formed by covalent bonds. A covalent bond involves a pair of electrons being shared between atoms.
A _____ covalent bond results when two atoms do not share the electrons equally so one end of the molecule assumes partial negative charge and the other end assumes a partial positive charge.
What is the similarity ratio of δabc to δdef. When two pairs of electrons are shared between two atoms a ____ bond is formed. What is the bond called that shares electrons between two neutral atoms. In a single covalent bond a single electron is shared between two atoms while in a double covalent bond two pairs of electrons are shared between two atoms. Covalent bond involves a pair of electrons being shared between atoms.
 Source: sciencedirect.com
Source: sciencedirect.com
Examples include the. A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. Students are also searching for. A ____ bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atoms.
The important concept to take from this is that in covalent bonds electrons in the outermost valence shell are shared to fill the valence shells of both atoms ultimately stabilizing both of the atoms involved.
A ____ bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. Examples include the. The chemical bond formed when two atoms share electrons between them is known as a covalent bond. When two pairs of electrons are shared between two atoms a ___ bond is formed.
 Source: sciencedirect.com
Source: sciencedirect.com
Instead it shares two electrons. The chemical bond formed when two atoms share electrons between them is known as a covalent bond. Covalent bonds are particularly common in organic materials where molecules often contain long chains of carbon atoms which have four electrons in their valence shells. 105 m 123 m 183 m 214 m.
 Source: mdpi.com
Source: mdpi.com
When one pair of electrons is shared between two atoms a ____ bond is formed. What is a bond called the shares electrons between two neutral atoms. A covalent bond forms when the difference between the electronegativities of two atoms is too small for. Shared electrons located in the space between the two nuclei are called bonding electrons.
 Source: sciencedirect.com
Source: sciencedirect.com
A covalent bond forms when the difference between the electronegativities of two atoms is too small for. Imagine a handshake between one hand an electron each from two people atoms. As long as the handshake holds it. What is the length of.
What is the bond called that shares electrons between two neutral atoms. Students are also searching for. When one pair of electrons is shared between two atoms a ____ bond is formed. As long as the handshake holds it.
The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases.
Examples include the. A Polar Covalent Bond is created when the shared electrons between atoms are not equally shared. What is the bond called that shares electrons between two neutral atoms. The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases. Bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms.
 Source: nature.com
Source: nature.com
Shared electrons located in the space between the two nuclei are called bonding electrons. Ionic bond 2 See answers. Metallic bond covalent bond ionic bond polar bond in Chemistry if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions. Examples include the. Metallic bond covalent bond.
Types of Covalent Bonds. Round to the nearest tenth. Polar _____ is a term used to describe an electrically neutral molecule formed by covalent bonds between atoms that have the same or similar electronegativity. What is it called when two atoms share two pairs of electrons.
An atom that loses one or more valence electrons to become a positively charged ion is known as a cation while an atom that gains electrons and becomes negatively charged is known as an anion.
An atom that loses one or more valence electrons to become a positively charged ion is known as a cation while an atom that gains electrons and becomes negatively charged is known as an anion. Atoms form covalent bonds in order to reach a more stable state. A bond in which two atoms share two pairs of electrons. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
 Source: mdpi.com
Source: mdpi.com
As long as the handshake holds it. Polar _____ is a term used to describe an electrically neutral molecule formed by covalent bonds between atoms that have the same or similar electronegativity. The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases. Ionic bond 2 See answers.
 Source: sciencedirect.com
Source: sciencedirect.com
What is a bond called that shares electrons between two neutral atoms. What is the similarity ratio of δabc to δdef. The important concept to take from this is that in covalent bonds electrons in the outermost valence shell are shared to fill the valence shells of both atoms ultimately stabilizing both of the atoms involved. The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases.
Find an answer to your question What is a bond called that shares electrons between two neutral atoms. This exchange of valence electrons allows ions to achieve electron configurations that mimic. A ____ bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. A covalent bond forms when the difference between the electronegativities of two atoms is too small for.
When one pair of electrons is shared between two atoms a ____ bond is formed.
A covalent bond is a chemical bond that involves the sharing of electron pairs between atomsThese electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Shared electrons located in the space between the two nuclei are called bonding electrons. As a result one end of the molecule has a slightly negative charge and the other a slightly positive charge. Is an electron pair shared between two atoms. The chemical bond formed when two atoms share electrons between them is known as a covalent bond.
 Source: sciencedirect.com
Source: sciencedirect.com
Atoms form covalent bonds in order to reach a more stable state. Instead it shares two electrons. The type of the chemical bond that forms when electrons are shared between atoms that are neutral have no electrical charge is A. An atom that loses one or more valence electrons to become a positively charged ion is known as a cation while an atom that gains electrons and becomes negatively charged is known as an anion. Metallic bond covalent bond.
The important concept to take from this is that in covalent bonds electrons in the outermost valence shell are shared to fill the valence shells of both atoms ultimately stabilizing both of the atoms involved.
Metallic bond covalent bond. This occurs when one atom has a higher electronegativity than the atom it is sharing with. An atom that loses one or more valence electrons to become a positively charged ion is known as a cation while an atom that gains electrons and becomes negatively charged is known as an anion. 105 m 123 m 183 m 214 m.
 Source: sciencedirect.com
Source: sciencedirect.com
This exchange of valence electrons allows ions to achieve electron configurations that mimic. This electron exchange results in an electrostatic attraction between the two atoms called an ionic bond. The sharing of electrons between the two atoms takes place in such a way that both the atoms acquire the stable electronic configurations of their nearest noble gases. Types of Covalent Bonds.
 Source: britannica.com
Source: britannica.com
A given nonmetal atom. AliceCooper AliceCooper 10132016 Chemistry High School answered What is a bond called that shares electrons between two neutral atoms. The chemical bond formed when two atoms share electrons between them is known as a covalent bond. Covalent bond involves a pair of electrons being shared between atoms.
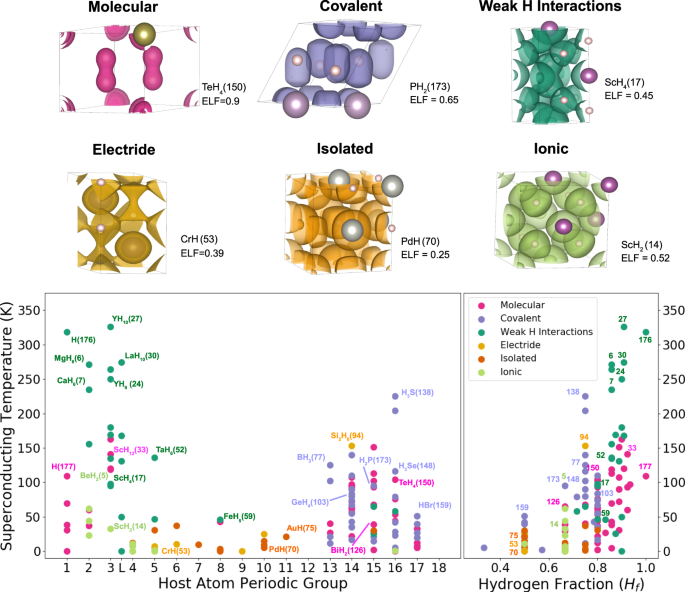 Source: nature.com
Source: nature.com
Such a shared pair of electrons is called a covalent Koh-VAY-lunt bond. Round to the nearest tenth. A _____ covalent bond results when two atoms do not share the electrons equally so one end of the molecule assumes partial negative charge and the other end assumes a partial positive charge. This exchange of valence electrons allows ions to achieve electron configurations that mimic.
Imagine a handshake between one hand an electron each from two people atoms.
A _____ covalent bond results when two atoms do not share the electrons equally so one end of the molecule assumes partial negative charge and the other end assumes a partial positive charge. Bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. The chemical bond formed when two atoms share electrons between them is known as a covalent bond. When two atoms come near each other they can share a pair of outermost electrons think of the atoms as tossing the electrons back and forth between them to form a covalent bond. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding.
 Source: mdpi.com
Source: mdpi.com
Metallic bond covalent bond. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions. A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. This exchange of valence electrons allows ions to achieve electron configurations that mimic. Types of Covalent Bonds.
Is an electron pair shared between two atoms.
What is a bond called that shares electrons between two neutral atoms. A ____ bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. Ionic bonds is a type chemical bond formed through an electrostatic attraction between two opposite charged ions. Imagine a handshake between one hand an electron each from two people atoms.
 Source: jbc.org
Source: jbc.org
The important concept to take from this is that in covalent bonds electrons in the outermost valence shell are shared to fill the valence shells of both atoms ultimately stabilizing both of the atoms involved. Bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. What is a bond called the shares electrons between two neutral atoms. Polar _____ is a term used to describe an electrically neutral molecule formed by covalent bonds between atoms that have the same or similar electronegativity. This occurs when one atom has a higher electronegativity than the atom it is sharing with.
 Source: pubs.rsc.org
Source: pubs.rsc.org
Ionic bond 2 See answers. Find an answer to your question What is a bond called that shares electrons between two neutral atoms. This exchange of valence electrons allows ions to achieve electron configurations that mimic. A given nonmetal atom. In a single covalent bond a single electron is shared between two atoms while in a double covalent bond two pairs of electrons are shared between two atoms.
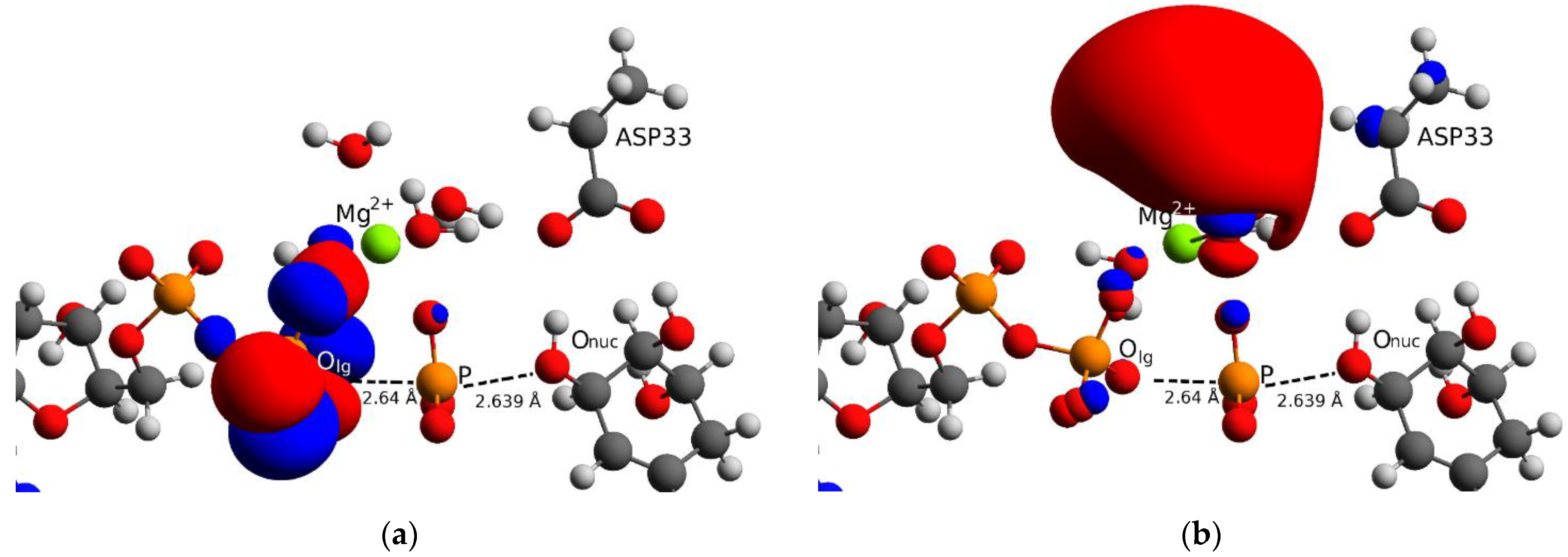 Source: mdpi.com
Source: mdpi.com
AliceCooper AliceCooper 10132016 Chemistry High School answered What is a bond called that shares electrons between two neutral atoms. The important concept to take from this is that in covalent bonds electrons in the outermost valence shell are shared to fill the valence shells of both atoms ultimately stabilizing both of the atoms involved. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions. Atoms form covalent bonds in order to reach a more stable state. This exchange of valence electrons allows ions to achieve electron configurations that mimic.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your favorite social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title what is a bond called that shares electrons between two neutral atoms by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.





