Which of the following atoms can attract a hydrogen atom in a hydrogen bond.
If you’re searching for which of the following atoms can attract a hydrogen atom in a hydrogen bond pictures information connected with to the which of the following atoms can attract a hydrogen atom in a hydrogen bond topic, you have come to the ideal blog. Our site frequently provides you with hints for refferencing the maximum quality video and picture content, please kindly surf and locate more enlightening video content and graphics that match your interests.
 Structure And Bonding 2 43 Hydrogen Bonding From ibchem.com
Structure And Bonding 2 43 Hydrogen Bonding From ibchem.com
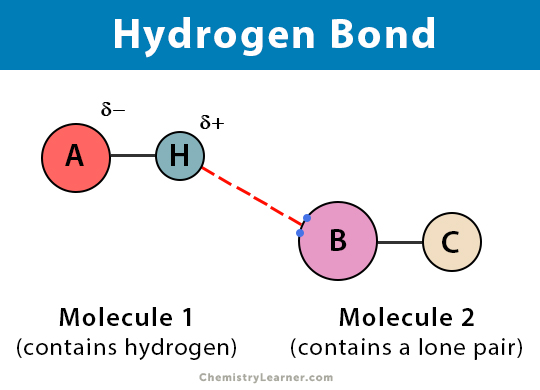
Answer 1 of 13. The first way gives rise to what is called an ionic bond. The hydrogen atoms are bound to the highly electronegative oxygen atom which also possesses two lone pair sets of electrons making for a very polar bond. A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen atom which is covalently bound to a more electronegative atom or group and another electronegative atom bearing a lone pair of electronsthe hydrogen bond acceptor.
A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen atom which is covalently bound to a more electronegative atom or group and another electronegative atom bearing a lone pair of electronsthe hydrogen bond acceptor.
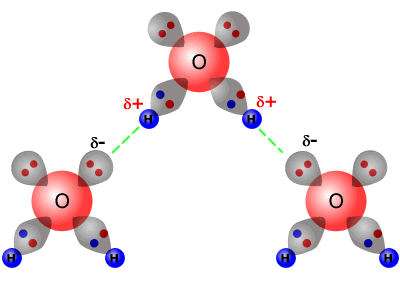
The bond between the H of one water molecule and the O of another water molecule b. Two hydrogen atoms b. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. There are three basic ways that the outer electrons of atoms can form bonds. These can then attract one another and create substances with higher boiling points.
 Source: pinterest.com
Source: pinterest.com
Which of the following is an example of a hydrogen bond. It results fromthe attractive force between a hydrogen atom covalently bonded toa very electronegative atom such as a N O or F atom and another veryelectronegative atom. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen. The attractive force which binds hydrogen atom of one molecule with electronegative atom such as fluorine oxygen and nitrogen of another molecule generally of the same substance is known as hydrogen bond. A hydrogen bond is a primarily electrostatic force of attraction between a hydrogen atom which is covalently bound to a more electronegative atom or group and another electronegative atom bearing a lone pair of electronsthe hydrogen bond acceptor.
As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds.
The bond between Na and Cl in salt e. The bond between C. Which of the following is an example of a hydrogen bond. Two hydrogen atoms b.
 Source: pinterest.com
Source: pinterest.com
The nitrogen atom is called the hydrogen bond acceptor because it is accepting the hydrogen from the oxygen. The only way these atoms can get closer to one another is if. The attractive force which binds hydrogen atom of one molecule with electronegative atom such as fluorine oxygen and nitrogen of another molecule generally of the same substance is known as hydrogen bond. A hydrogen atom bonded to F O or N is attracted to an electron pair.
 Source: pinterest.com
Source: pinterest.com
Answer 1 of 13. Hydrogen bond acceptor and the hydrogen itself can be as short as 18-19 Å well below the sum of the atomic radii eg. These can then attract one another and create substances with higher boiling points. A hydrogen atom in a molecule forms a bond with any atom.
 Source: chemistrylearner.com
Source: chemistrylearner.com
You know that the Lennard-Jones potential rises with 1r12 if we get closer than the Van der Vaals radii. The bond between C. Answer 1 of 13. Hydrogen bond acceptor and the hydrogen itself can be as short as 18-19 Å well below the sum of the atomic radii eg.
A hydrogen bond can form between_____ adjacent to each other. The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule see Figure below. Hydrogen bond strengths range from 4 kJ to50 kJ per mole of hydrogen bonds. Which of the following is an example of a hydrogen bond.
The bond between two hydrogen atoms d.
A hydrogen atom bonded to F O or N is attracted to an electron pair. Which of the following is an example of a hydrogen bond. Each hydrogen atom can form a hydrogen bond with a nitrogen fluorine or oxygen atom. A hydrogen atom forms a covalent bond with another atom. A hydrogen atom and an oxygen atom.

Two hydrogen atoms b. The first way gives rise to what is called an ionic bond. Atom - atom - Atomic bonds. As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds. This sums to four hydrogen bonds per water molecule.
The bond between the H of one water molecule and the O of another water molecule b. These can then attract one another and create substances with higher boiling points. The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule see Figure below. A hydrogen atom bonded to F O or N is attracted to an electron pair.
There are three basic ways that the outer electrons of atoms can form bonds.
The elements that usually participate in hydrogen bonds are nitrogen oxygen and fluorine. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. A hydrogen atom forms a covalent bond with another atom. The first way gives rise to what is called an ionic bond.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Hydrogen bond strengths range from 4 kJ to50 kJ per mole of hydrogen bonds. Each hydrogen atom can form a hydrogen bond with a nitrogen fluorine or oxygen atom. The bond between Mg and Cl in MgCl2 c. The bond between two hydrogen atoms d.
 Source: pinterest.com
Source: pinterest.com
Hydrogen bond strengths range from 4 kJ to50 kJ per mole of hydrogen bonds. Which of the following is an example of a hydrogen bond. For example several H-F molecules are associated by hydrogen bond s as. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen.
 Source: slidetodoc.com
Source: slidetodoc.com
Once the way atoms are put together is understood the question of how they interact with each other can be addressedin particular how they form bonds to create molecules and macroscopic materials. The elements that usually participate in hydrogen bonds are nitrogen oxygen and fluorine. A hydrogen bond can form between_____ adjacent to each other. A hydrogen atom bonded to F O or N is attracted to an electron pair.
As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds.
A hydrogen atoms form an ionic bond with another atom on an adjacent molecule. A hydrogen bond can form between_____ adjacent to each other. As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds. This sums to four hydrogen bonds per water molecule. A hydrogen atoms form an ionic bond with another atom on an adjacent molecule.
 Source: adichemistry.com
Source: adichemistry.com
In molecules containing N-H O-H or F-H bonds the large difference inelectronegativity between the H atom and the N O or F atom leads to ahighly polar. For example several H-F molecules are associated by hydrogen bond s as. The bond between two hydrogen atoms d. The bond between C. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen.
As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds.
A hydrogen atom in a molecule forms a bond with any atom. It results fromthe attractive force between a hydrogen atom covalently bonded toa very electronegative atom such as a N O or F atom and another veryelectronegative atom. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen. A hydrogen atom in a molecule forms a bond with any atom.
 Source: pinterest.com
Source: pinterest.com
The first way gives rise to what is called an ionic bond. Which of the following is an example of a hydrogen bond. The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule see Figure below. Once the way atoms are put together is understood the question of how they interact with each other can be addressedin particular how they form bonds to create molecules and macroscopic materials.
 Source: wikihow.com
Source: wikihow.com
A hydrogen atom bonded to F O or N is attracted to an electron pair. You know that the Lennard-Jones potential rises with 1r12 if we get closer than the Van der Vaals radii. 12Å for hydrogen and 15Å for oxygen and nitrogen. Answer 1 of 13.
 Source: pinterest.com
Source: pinterest.com
Each hydrogen atom can form a hydrogen bond with a nitrogen fluorine or oxygen atom. The nitrogen atom is called the hydrogen bond acceptor because it is accepting the hydrogen from the oxygen. A hydrogen atoms form an ionic bond with another atom on an adjacent molecule. The attractive force which binds hydrogen atom of one molecule with electronegative atom such as fluorine oxygen and nitrogen of another molecule generally of the same substance is known as hydrogen bond.
The bond between Mg and Cl in MgCl2 c.
You know that the Lennard-Jones potential rises with 1r12 if we get closer than the Van der Vaals radii. It results fromthe attractive force between a hydrogen atom covalently bonded toa very electronegative atom such as a N O or F atom and another veryelectronegative atom. Once the way atoms are put together is understood the question of how they interact with each other can be addressedin particular how they form bonds to create molecules and macroscopic materials. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen. The only way these atoms can get closer to one another is if.
 Source: ibchem.com
Source: ibchem.com
A hydrogen atom in a molecule forms a bond with any atom. Answer 1 of 13. The first way gives rise to what is called an ionic bond. You know that the Lennard-Jones potential rises with 1r12 if we get closer than the Van der Vaals radii. Hydrogen bond is formed only by the three highly electronegative elements- fluorine oxygen and nitrogen.
Two hydrogen atoms b.
The bond between Mg and Cl in MgCl2 c. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. Which of the following is an example of a hydrogen bond. The partially positive hydrogen atom of one molecule is then attracted to the oxygen atom of a nearby water molecule see Figure below.
 Source: ibchem.com
Source: ibchem.com
This sums to four hydrogen bonds per water molecule. There are three basic ways that the outer electrons of atoms can form bonds. Answer 1 of 13. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. As these three atoms are the only ones with sufficient electronegativity that when bound to hydrogen can form hydrogen bonds.
 Source: slidetodoc.com
Source: slidetodoc.com
Two hydrogen atoms b. The bond between the H of one water molecule and the O of another water molecule b. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. The only way these atoms can get closer to one another is if. Two hydrogen atoms b.

The first way gives rise to what is called an ionic bond. In the picture of two water molecules at lower right the oxygen of the water molecule B is the hydrogen bond donor. Each hydrogen atom can form a hydrogen bond with a nitrogen fluorine or oxygen atom. The attractive force which binds hydrogen atom of one molecule with electronegative atom such as fluorine oxygen and nitrogen of another molecule generally of the same substance is known as hydrogen bond. Atom - atom - Atomic bonds.
This site is an open community for users to submit their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which of the following atoms can attract a hydrogen atom in a hydrogen bond by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.